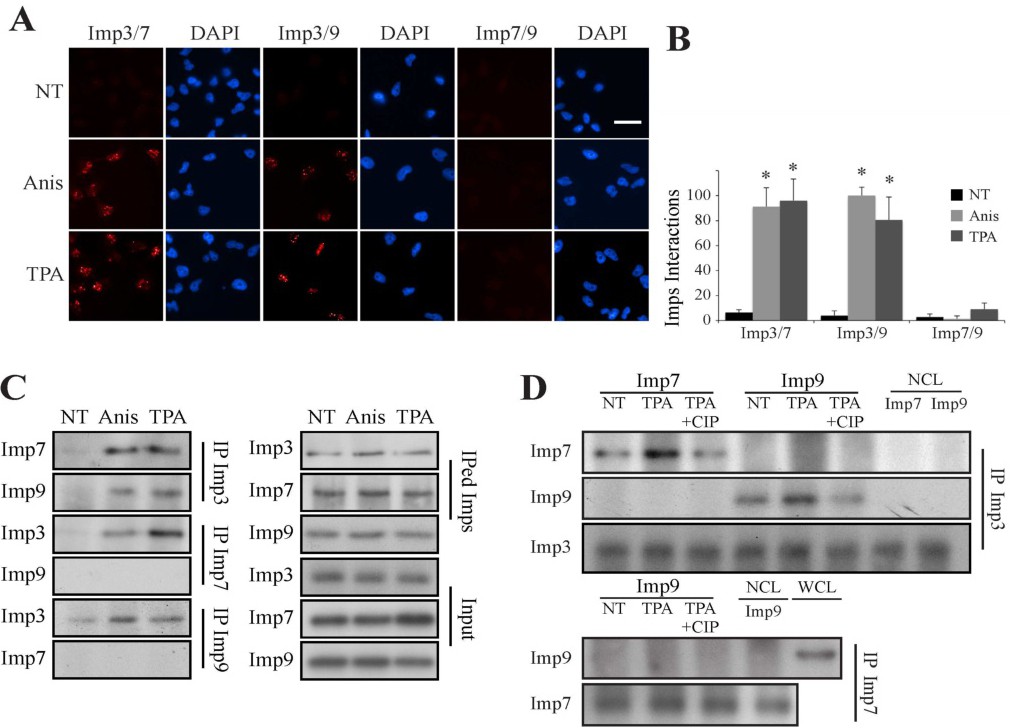
Fig. 9. Imp3 interacts with Imp7/9-MAPK dimer in a phospho-dependent manner. (A) Proximity ligation assay demonstrates stimulus-dependent interaction of Imp3 with Imp7/9. HeLa cells were grown on slides to 70% confluence, serum starved and either stimulated with anisomycin (Anis, 0.5 Ág/ml, 15 min) or left untreated (NT). The cells were then fixed and subjected to a PLA using the indicated Abs and visualized using a fluorescent microscope. The bar in the upper right panel is of 20 ÁM. (B) Quantification of the intensity of the signals was performed using ImageJ. The data shown represents mean ▒ S.E of three different experiments (* - p<0.0005). (C) CoIP assay confirms the interaction of Imp3/7 and Imp3/9. Serum-starved HeLa cells were stimulated with either anisomycin (Anis 0.5 μg/ml, 15 min), TPA (250 nM, 15 min) or left untreated (NT). Cells extracts were then subjected to CoIP with indicated Abs. Imps, IPed Imps and inputs were detected by Western blot with the indicated Abs. (D) The interaction of Imp3 with Imp7/9 is dependent on Imp3 phosphorylation. IPed Imp3 (upper panels) or Imp7 (lower panels) were purified from either stimulated or untreated cells, as described. Purified Imps were then incubated with calf intestinal phosphatase, (CIP, 1 hr, 37░C) or left untreated followed by washing. Recombinant Imps were incubated with the IPed Imps (2 hr, 37░C) and then washed. The interacting Imps were detected using Western blotting with the indicated Abs (NCL - no cell lysate, WCL - whole cell lysate). The experiments were reproduced twice.